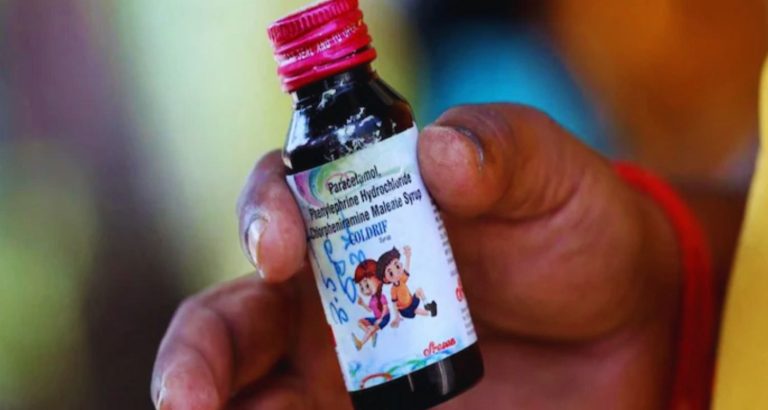
The World Health Organization (WHO) has issued a global alert over three toxic cough syrups manufactured in India, which have been linked to the deaths of several children in Madhya Pradesh.
Laboratory tests confirmed that the syrups, Coldrif, Respifresh TR, and ReLife, contained dangerously high levels of diethylene glycol (DEG), a toxic chemical often used as an industrial solvent. Ingestion of DEG can cause acute kidney injury and death, particularly in children.
According to WHO, the contaminated syrups were produced by a company based in Madhya Pradesh and have been associated with the deaths of multiple children under the age of five in Chhindwara district.
India’s Central Drugs Standard Control Organization (CDSCO) and state health authorities have since recalled the affected batches and halted manufacturing operations at the implicated facilities.
The health agency warned that the syrups pose a “serious risk to public health,” urging parents, health workers, and regulators to stop their use immediately. So far, there is no evidence the contaminated products were exported outside India.
The WHO alert follows an investigation by Indian health officials after several children reportedly developed acute kidney failure after consuming the syrups.
This is not the first time India’s pharmaceutical sector has come under scrutiny. In recent years, WHO has linked contaminated cough syrups made in India to child deaths in The Gambia, Uzbekistan, and Cameroon, prompting calls for stricter drug quality control.
Authorities in Madhya Pradesh have begun a criminal investigation into the manufacturer, while WHO continues to monitor for potential international distribution of the toxic products.